Latest Research
From nanospace to global problem solving
PROFESSOR. Norikazu Nishiyama
~Building a carbon-neutral manufacturing process for a sustainable society~

Reaction engineering is the discipline that elucidates the reaction mechanism from a microscopic viewpoint at the molecular level, aiming to utilize the results to design an optimal reaction device from a macroscopic viewpoint. When you hear the word “reactor,” you may imagine a big one, but in my research, I use a micromolecular-level space called nanospace as a reaction device instead of a visible device. We are engaged in research to promote the conversion and separation of substances by using “nanospace materials” that are effective as chemical devices as catalysts, adsorbents, and separation membrane materials.
Converting “nanospace materials” into chemical reaction devices by making unique ingenuity
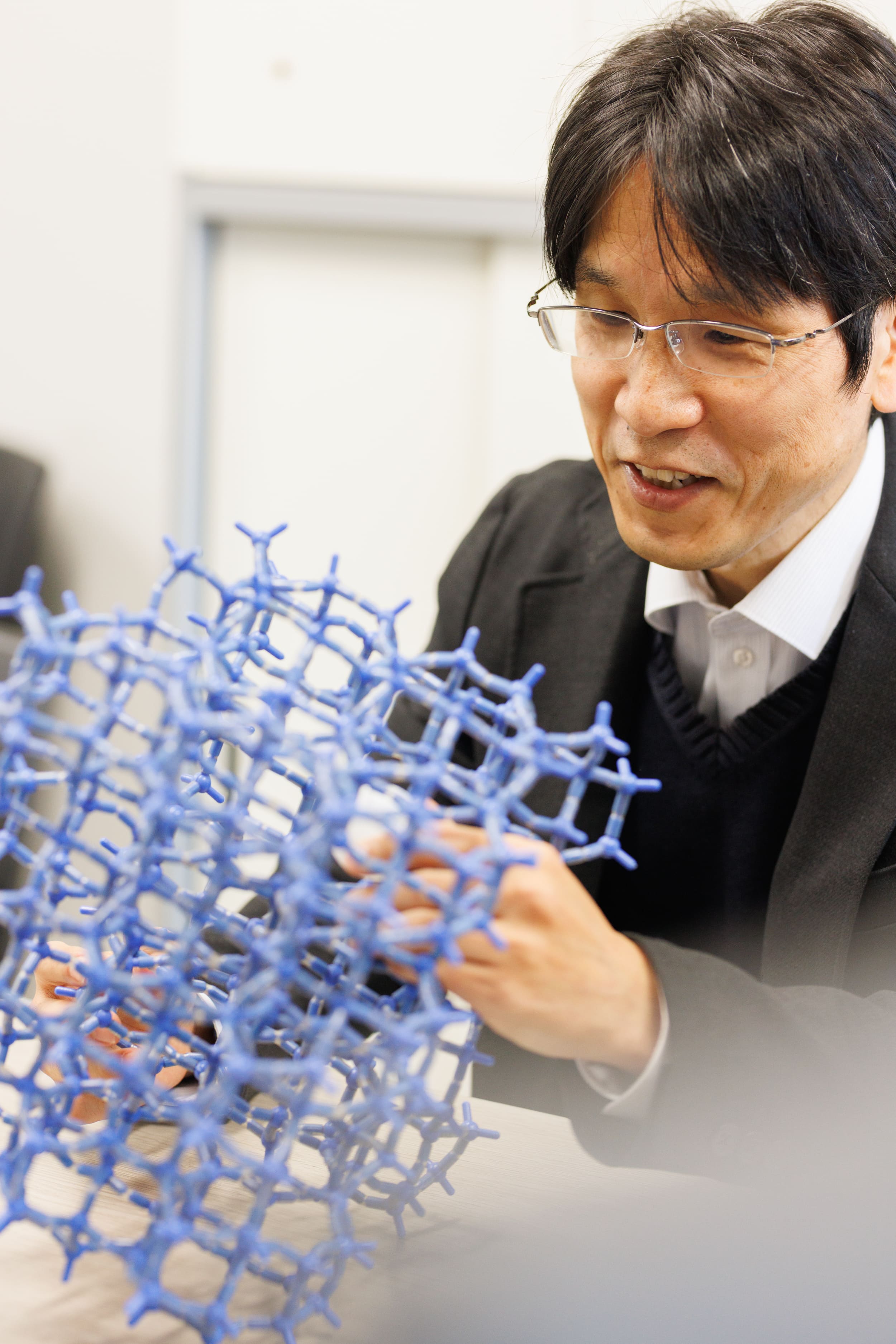
 The nanospace material
that I mainly use as a catalyst is a mineral called zeolite. There are more than 100 types of zeolite
crystal structures, and they have a wide variety of molecular-level nanospaces. It has a function to
selectively incorporate into nanospace according to the molecular size and shape (Molecular Sieve, Shape
Selectivity). In other words, the molecule to be accepted is determined by the type of zeolite. Therefore,
how to design a zeolite (nanospace) that suits the purpose is an important issue. Then, how does the
nanospace designed and synthesized in this way become a chemical reaction device, and what kind of process
does it take to generate new substances? Let’s take an example of process of producing para-xylene, which is
the raw material for PET bottles, from carbon dioxide. First, carbon dioxide is separated and concentrated
using nanospace materials. For that purpose, it is necessary to develop a porous body in which nanospaces
with high affinity for carbon dioxide are lined up. We also working on morphological control of nanospace
materials, for example, by thinning the membrane, it will be possible to continuously separate carbon
dioxide. Carbon dioxide is converted to methanol, which is one of key substances in manufacturing. Next,
methanol undergoes several reactions in the nanospace of zeolite to become paraxylene. In other words, the
first key is that both methanol and paraxylene have the right molecular size to pass through the nanospace.
The nanospace material
that I mainly use as a catalyst is a mineral called zeolite. There are more than 100 types of zeolite
crystal structures, and they have a wide variety of molecular-level nanospaces. It has a function to
selectively incorporate into nanospace according to the molecular size and shape (Molecular Sieve, Shape
Selectivity). In other words, the molecule to be accepted is determined by the type of zeolite. Therefore,
how to design a zeolite (nanospace) that suits the purpose is an important issue. Then, how does the
nanospace designed and synthesized in this way become a chemical reaction device, and what kind of process
does it take to generate new substances? Let’s take an example of process of producing para-xylene, which is
the raw material for PET bottles, from carbon dioxide. First, carbon dioxide is separated and concentrated
using nanospace materials. For that purpose, it is necessary to develop a porous body in which nanospaces
with high affinity for carbon dioxide are lined up. We also working on morphological control of nanospace
materials, for example, by thinning the membrane, it will be possible to continuously separate carbon
dioxide. Carbon dioxide is converted to methanol, which is one of key substances in manufacturing. Next,
methanol undergoes several reactions in the nanospace of zeolite to become paraxylene. In other words, the
first key is that both methanol and paraxylene have the right molecular size to pass through the nanospace.
Another important point is that it is necessary to connect the nanospaces of zeolite. Paraxylene is one of the three isotopes of xylene. Some of the generated para-xylene will be converted to another xylene near the exit of the nanospace. Therefore, we constructed a space in which para-xylene is selectively generated by connecting a non-catalytic nanospace to the exit of the nanospace and adding a function.
The development of new technologies using these nanospace materials is also being carried out as part of the NEDO (New Energy and Industrial Technology Development Organization) project. We are also exploring the establishment of chemical recycling technology that uses zeolite to decompose waste plastic to the molecular level and recycle it. These technologies are essential for achieving carbon neutrality for a sustainable society.
The magnificent scale conversion from micro to macro is attractive
Currently, in addition to the NEDO project, we are conducting research in collaboration with emerging companies that are trying to start a new chemical recycling business. In this way, the number of collaborative research related to the environmental field is increasing in this era. So far, I have been interested in how to create a nanospace or what happens in that space in my research. I think that it is one of the research strengths of engineering science: my interest in working on it can greatly contribute to solving social problems. The chemical reactions that occur in the invisible small world (nanospace) of 10-9 will lead to the construction of sustainable society, which is a global issue. The real pleasure about this research could be its extraordinary scale: we can reach the value of 10 raised to the power of X from nano.
Nanospace materials are a remarkable research field in which new properties and phenomena are being discovered one after another due to the diversity of target substances and structures. I would like to seek further possibilities so that I can conduct research that meets the needs of that era.
