Press Release
Artificial intelligence (AI) discovers order in the disordered structure of glass!
Key Findings
・AI discovers order in the bond between molecules hidden within the disordered molecular arrangement of glass.
・It has been difficult to distinguish glass from liquids because they both retain the same disordered structure, but it has become possible to identify glass by using deep learning of data of molecular arrangement structure.
・Expect progress in AI technology that views glass as a phenomenon of molecular congestion and predicts and clarifies physical properties such as glass transition temperature from structural data on disordered molecular arrangements.
Outlines
A research group including Kohei Yoshikawa (master course), Associate Professor Kang Kim, and Professor Nobuyuki Matsubayashi of the Graduate School of Engineering Science at the University of Osaka discovered that deep learning of the molecular arrangement structure of glass can identify the order hidden in the bonds between molecules within a disordered structure.
Glass is a common material in our daily lives. At first glance, it appears to be solid, but when looking at its molecular structure, it has the same irregular arrangement as liquids, unlike solids such as crystals, which have a regular arrangement. It is known that if the constituent molecules are changed, even the same glass can have significantly different physical properties, such as glass transition temperature.
Therefore, it is necessary to elucidate the laws behind the amorphous structure in order to clarify the properties common to glass.
To turn a substance into a glass, the liquid must be supercooled below its melting point to freeze the liquid's disordered structure. It means putting the molecules in a congested state. Therefore, it will be the key to clarify how the disordered structure changes as the temperature of the supercooled liquid decreases. On the other hand, the change was subtle, making it difficult to distinguish between liquid and glass.
In this study, the research group used molecular arrangement structures which were obtained through computer simulations called molecular dynamics as input data as well as a deep learning method called Graph Neural Networks (GNNs) to elucidate the subtle differences in disordered structures caused by temperature differences. (Fig. 1)
This result demonstrates the AI has detected the fact that there is order in the disordered structure. The research group also used a method called the attention mechanism, which characterizes the importance of input data, to provide a physical interpretation for why the AI demonstrated such high predictive performance in a way that is understandable to humans. Thanks to this, it is expected to lead to the progress of technology that utilizes AI to clarify the laws that govern the disordered molecular arrangement of glass, and to predict its physical properties known as molecular congestion, from its molecular arrangement.
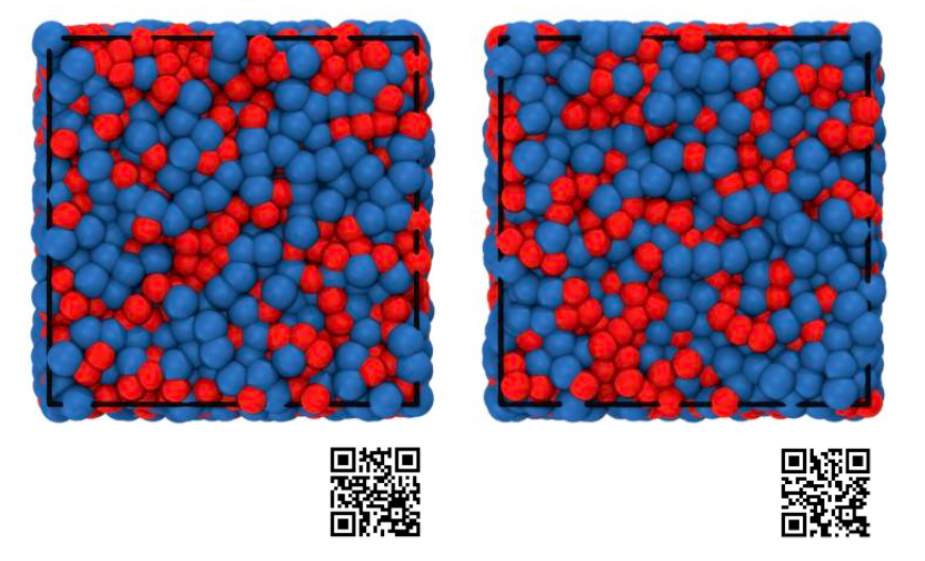
Fig. 1: The disordered molecular structure obtained through computer simulations in a liquid state above the melting point (left) and in a state immediately before supercooling and vitrification (right). Humans cannot tell the difference in temperature, but the AI was able to distinguish slight differences in the bonds between molecules and accurately predict the difference in temperature.
Credit: Kang Kim
Research Background
Glass behaves like a solid while retaining the same disordered structure as liquids. Therefore, elucidating the factors that cause the glass transition, a phenomenon in which the disordered structure of glass freezes apparently, is a challenge in materials science and has fascinated many researchers. In order to study glass, it is necessary to clarify the properties of supercooled liquids, which are supercooled below their melting point. There, computer simulations known as the molecular dynamics method is used to perform theoretical analysis. Molecular dynamics method generates a huge amount of coordinated data related to molecular arrangements from the interactions between molecules. By analyzing this data based on statistical mechanics, it is possible to clarify how the viscosity increases rapidly as the temperature decreases toward the glass transition temperature, and how the disordered structure freezes. Various studies have been conducted on this topic so far. On the other hand, the structural changes related to temperature are very slight, making it difficult to find the order that causes the glass transition.
Research Contents
The research group revealed that a deep learning method called the Graph Neural Networks (GNNs) can distinguish between the subtle structural differences between liquids and glass at different temperatures. In particular, by representing molecules as nodes and the interrelations between molecules as edges in a graph structure, it takes advantage of the benefits of deep learning, which fully utilizes the structure of disordered molecular arrangements as input data (Fig. 2). This means the AI found the hidden order in the disordered structure of the glass. Furthermore, by applying a technology called the attention mechanism, which clarifies what parts of the input data of a neural network should be paid attention to, the researchers succeeded in quantifying the degree of attention of surrounding molecules to a certain molecule. As a result, they revealed the AI determines the degree of order on a molecular-size spatial scale for each molecule and predicts its temperature.
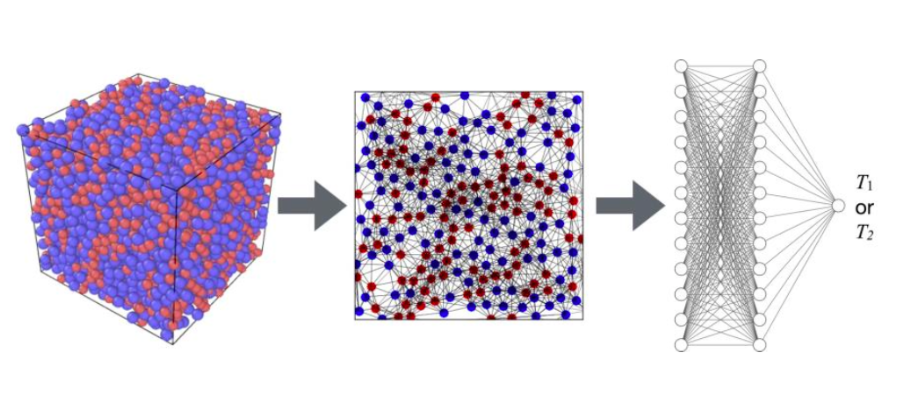
Fig. 2: From molecular coordinate data, a graph structure is created that represents molecules as nodes and the interrelation between molecules as edges. By using deep learning to understand the number of features that retain the graph information, the temperature of the input structure is predicted.
Credit: Kang Kim
Social Impact of this Research Result
The results of this research showed that deep learning can detect hidden order even in disordered molecular structures. This suggests that it is possible to elucidate how molecules congest in glass and how they get out of it. In fact, it is known that molecular mobility is completely different depending on the temperature.
In a liquid above its melting point, molecules can move almost freely, but when the liquid is packed tightly together and approaches glass, the molecules cannot move freely and try to cooperate with each other. The QR code in Fig. 1 shows the movement of molecules in a computer simulation. It's just like the movements of people on a crowded train. If AI can learn how to resolve congestion by utilizing order from information on molecular arrangement, it may be possible to clarify, for example, the factors behind the large differences in glass transition temperatures that occur depending on the molecules that make up a substance.
Notes
The article, “Graph neural network-based structural classification of glass-forming liquids and its interpretation via self-attention mechanism,” was published in Journal of Chemical Physics at DOI: https://doi.org/10.1063/5.0277279
Links
Associate Professor Kim’s Profile
Professor Matsubayashi’s Profile
Please see ResOU for the detail.